Galvanic corrosion occurs between two metals with different oxidation potentials, such as steel and copper. However, other conditions are also required, thanks to which Apliweld® Secure+ aluminothermic welding does not oxidise the corrugated steel to copper cable connection.
Corrosion is a destructive attack of a material by reaction with its environment. The consequences of corrosion result in major problems such as plant shutdowns, loss of valuable resources, product contamination, reduced efficiency and costly maintenance. They can also compromise safety and inhibit technological progress1.
Corrosion controlling requires the understanding of its mechanisms. It also requires the use of corrosion-resistant designs and materials, or protective systems, devices, and treatments1.
In this text, the oxidation of a steel structure when using Apliweld® Secure+ aluminothermic welding is presented qualitatively. The quantitative analysis depends on the individual case and is therefore not covered in this article of a more general nature.
Galvanic corrosion of metals
Galvanic corrosion occurs when two dissimilar metallic materials are brought into contact in the presence of an electrolyte or electrically conductive medium. This phenomenon can occur between metals and alloys, and other conductive materials such as carbon or graphite1.
Due to the differences in the corrosion potentials of different materials, an electrochemical corrosion cell1 is established, i.e. a galvanic cell or battery whose electrochemical reactions generate corrosion. The tendency of materials to be oxidised or reduced by another metal is determined by the galvanic series.
The greater distance in the galvanic series between two materials increases the risk of galvanic corrosion. However, this series does not provide information on the rate of corrosive attack. In addition, the relative position of the materials could change depending on the environment in which the metals are located1.
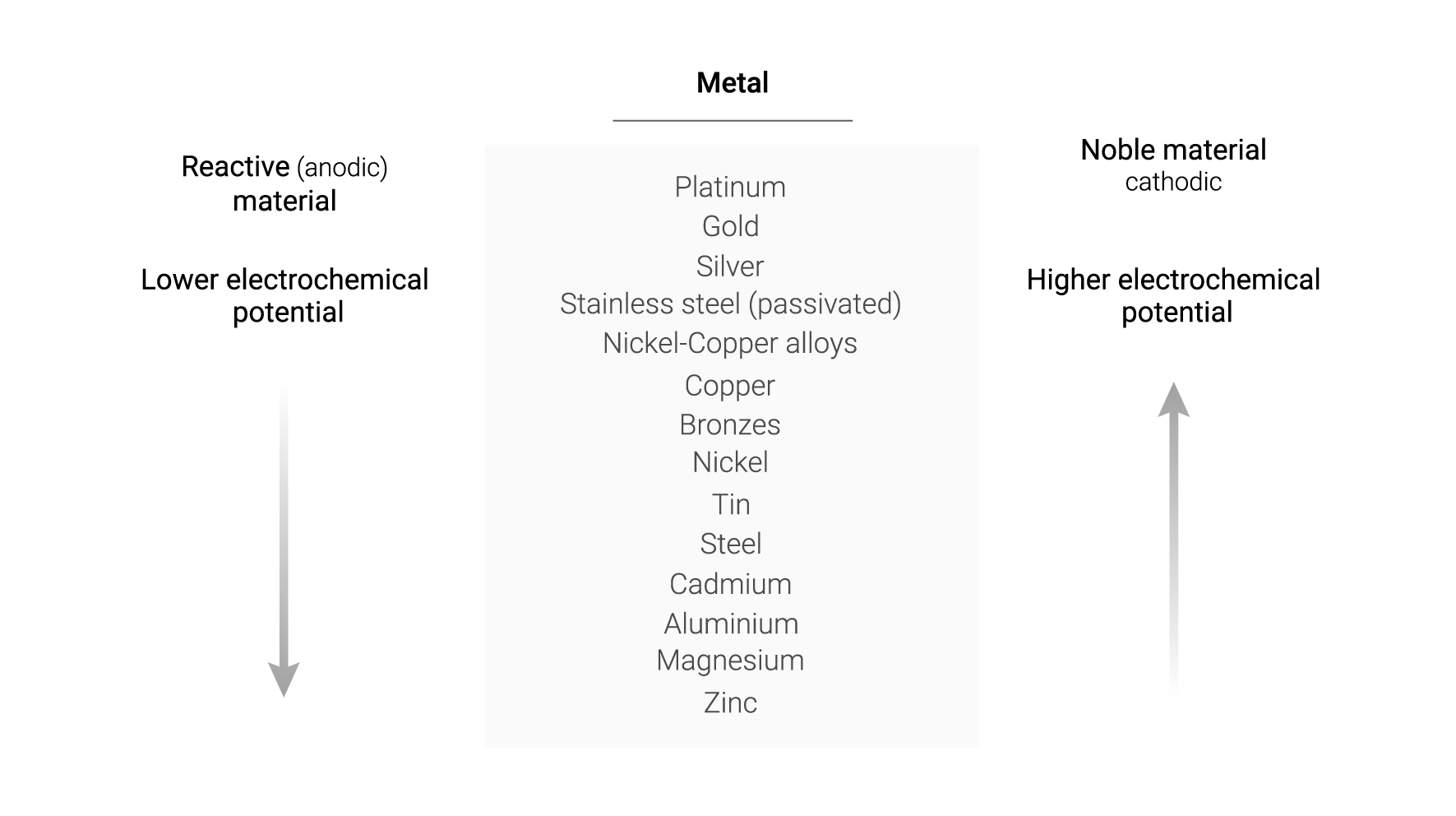 Following the table of the galvanic series, metals in higher rows will, in theory, oxidise those below them. For example, gold oxidises magnesium, silver oxidises cadmium, tin oxidises aluminium and, in this case, copper oxidises steel. The question could then arise as to whether, in Apliweld® Secure+ aluminothermic welding, the resulting copper oxidises steel structures. However, this is not the case and will be explained below after a brief description of the Apliweld® Secure+ welding system.
Following the table of the galvanic series, metals in higher rows will, in theory, oxidise those below them. For example, gold oxidises magnesium, silver oxidises cadmium, tin oxidises aluminium and, in this case, copper oxidises steel. The question could then arise as to whether, in Apliweld® Secure+ aluminothermic welding, the resulting copper oxidises steel structures. However, this is not the case and will be explained below after a brief description of the Apliweld® Secure+ welding system.
Aluminothermic welding Apliweld® Secure+
Copper aluminothermic welding, or exothermic welding, is mainly used in earthing systems in electrical installations. This type of welding solves the problems associated with mechanical joints with clamp connectors, such as human failure and quality deterioration over time.
This is because, unlike connector joints, copper aluminothermic welding is a chemical process that achieves a molecular and irreversible bond between copper, stainless steel, galvanised, bronze, etc. conductors. The basis of the process is an exothermic chemical reaction in which the aluminium reduces the copper oxide, producing the copper which totally or partially melts the conductors to be soldered. The result is a connection superior to any mechanical joint in terms of mechanical and electrical properties. Its conductivity is equal to or better than that of the conductors themselves, without increasing the resistance.
Apliweld® Secure+ is the exothermic welding system developed by Aplicaciones Tecnológicas, composed of charge in tablets, an electronic initiator and remote ignition (activated by Bluetooth). Apliweld® Secure+ is positioned in the market as the exothermic welding that offers more advantages for workers, with a particular focus on occupational safety, while allowing more accurate and efficient results of the joints in earthing systems. Apliweld® Secure+ is therefore the tool for any exothermic welding project.
Now we will discuss the particular case of the connection of corrugated steel to the copper cable using Apliweld® Secure+ exothermic welding.
Factors affecting galvanic corrosion
The galvanic series is only one of the conditions necessary for galvanic corrosion to take place, as several factors enable or prevent galvanic corrosion to occur. These include the presence of an electrolyte medium, the anodic and cathodic areas, the exposure time or the kinetics of the reaction itself. For a qualitative assessment, the first two are studied.
On the one hand, the electrolyte medium refers to the environment in which the metals are in contact. Thus, if there is no medium through which the current flows, corrosion cannot occur. With an electrolyte medium, on the other hand, corrosion occurs quantitatively depending on the conductivity of the medium, although other characteristics also play a role. Typically, deionised water is a poor conductor, whereas rainwater has a somewhat higher conductivity, and seawater is a clear case of a corrosive medium.
In addition, or in conjunction with the previous factor, the anodic and cathodic areas of the metals in contact are compared, i.e. the amount of material that oxidises as a function of the amount of oxidising material. Thus, if there is a large area of oxidising material relative to the metal that can be oxidised (always assuming a conductive electrolyte medium), corrosion will take place at a high rate. However, in the opposite case, oxidation does not occur or is negligible.
Corrugated steel to copper cable connection with Apliweld® Secure+ welding
Although according to the galvanic series copper should corrode corrugated steel, it is necessary to take into account the other factors presented.
Considering the connection itself made with Apliweld® Secure+ aluminothermic welding, there is no electrolyte medium inside the connection, so corrosion is impossible. In the wire/rod welding, it can be seen (as shown in the picture) the coexistence in the solid state of the molten filler copper and the wire itself, the unmelted steel, and the copper/steel alloy of the molten material. None of them forms an electrolyte medium to produce oxidation, so corrosion does not occur.
 On the other hand, there is the possibility of corrosion between the outside of the welding and the corrugated steel of the installation. In this case, the contact surface is minimal. Likewise, the electrolyte medium present is the concrete that will cover the connection and which is a poor conductor of current. In addition, the factor that makes corrosion most difficult is the much larger area of the corroding element (i.e. steel) concerning the surface of the oxidising metal (copper).
On the other hand, there is the possibility of corrosion between the outside of the welding and the corrugated steel of the installation. In this case, the contact surface is minimal. Likewise, the electrolyte medium present is the concrete that will cover the connection and which is a poor conductor of current. In addition, the factor that makes corrosion most difficult is the much larger area of the corroding element (i.e. steel) concerning the surface of the oxidising metal (copper).
Therefore, although the galvanic series indicates that Apliweld® Secure+ welding could cause corrosion on corrugated steel structures, the other factors necessary for corrosion to occur are either not present or not conducive to corrosion at all.
Therefore, the Apliweld® Secure+ welding does not corrode or, in case there is some corrosion, it cannot develop significantly. In this respect, it should be noted that any other type of connection (mechanical connection, welding, …) between steel and copper for an earthing network involves a higher possibility of corrosion than the Apliweld® Secure+ welding.
For more information about the advantages of Apliweld® Secure+ welding in terms of efficiency and safety, do not hesitate to contact our experts at the following link.
At Aplicaciones Tecnológicas we also offer free online training on aluminothermic welding which you can register for on the webinars page.
References
- Roberge, P. R. Handbook of corrosion engineering. McGraw-Hill Education Third Edition (2000).
